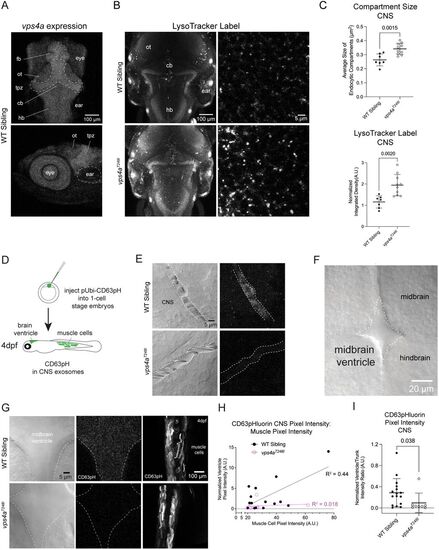
vps4aT248I mutants have enlarged endosomal compartments and release fewer exosomes in the CNS. A, Confocal Z-projections of a 5 dpf wild-type zebrafish larva labeled with HCR RNA-FISH probes for vps4a. fb, forebrain; ot, optic tectum; tpz, tectal proliferation zone, cb, cerebellum; hb, hindbrain. B, LysoTracker staining of 5 dpf wild-type siblings and vps4aT248I mutants show an increased staining in the mutant. C, Acidic membrane compartment size in the hindbrain of vps4aT248I mutants (N = 10) is significantly larger than those in wild-type siblings (N = 7); unpaired t test; p = 0.0015; t = 3.873; df = 15. LysoTracker vital dye taken up by vps4aT248I mutants (N = 10), measured as normalized pixel integrated density, is significantly higher compared with wild-type siblings (N = 7); unpaired t test; p = 0.0020; t = 3.744; df = 15. D, Schematic of injecting one-cell stage nacre-background embryos with pUbi:CD63-pHluorin. Healthy larvae were sorted on 1 dpf, and larvae expressing CD63-pHluorin in muscle cells was sorted on 3 dpf, for imaging on 4 dpf. E, Decreased CD63-pHluorin expression in 3 dpf vps4aT248I mutant CNS blood vessels. F, Dorsal view of 4 dpf zebrafish larva showing the midbrain ventricle. G, Decreased CD63-pHluorin expression in vps4aT248I mutant brain ventricles, but trunk muscles express comparable levels to wild-type siblings. H, Pixel intensity of muscles cells is correlated with normalized pixel intensity of CD63-pHluorin signal in the midbrain ventricle in wild-type siblings (N = 16). I, Quantification of CD63-pHluorin fluorescence in wild-type siblings (N = 16) and vps4aT248I mutants (N = 8); Mann–Whitney test; p = 0.0382 (exact).
|

