- Title
-
Pex1 loss-of-function in zebrafish is viable and recapitulates hallmarks of Zellweger spectrum disorders
- Authors
- Heins-Marroquin, U., Hodzic, Z., da Silva, B.S.C., Hendriks, A., Gavotto, F., Warmoes, M.O., Schlicker, L., Omri, S., Jäger, C., Glaab, E., Braverman, N.E., Cordero-Maldonado, M.L., Linster, C.L.
- Source
- Full text @ Front. Mol. Neurosci.
|
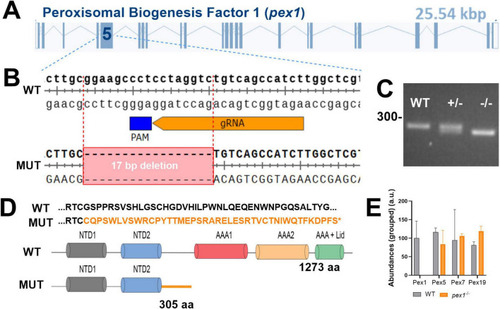
Generation of a |
|
Survival and phenotypic characterization of the PHENOTYPE:
|
|
Peroxisome biogenesis is compromised in adult |
|
Liver steatosis and lipid dyshomeostasis manifest during early development in |
|
Transcriptomics analysis of |
|
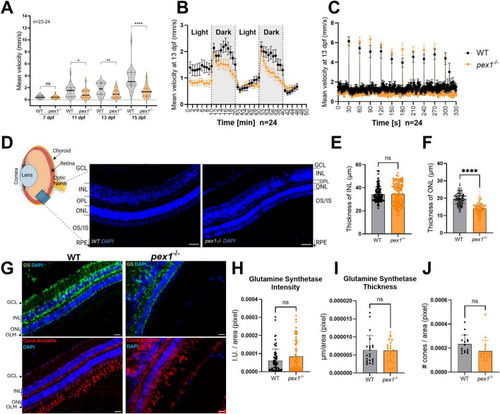
Pex1 deficiency leads to behavioral changes in larvae and perturbed retinal architecture in adult fish. |
|
Schematic overview of the main phenotypic outcomes observed in the PHENOTYPE:
|