- Title
-
Transmembrane Protein-184A Interacts with Syndecan-4 and Rab GTPases and Is Required to Maintain VE-Cadherin Levels
- Authors
- Altenburg, L.M., Wang, S.H., Ciabattoni, G.O., Kennedy, A., O'Toole, R.L., Farwell, S.L.N., Iovine, M.K., Lowe-Krentz, L.J.
- Source
- Full text @ Cells
|
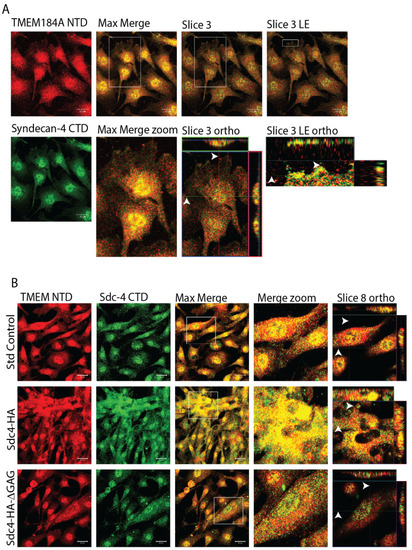
TMEM184A interacts with Sdc4 proteoglycans in BAOECs. ( |
|
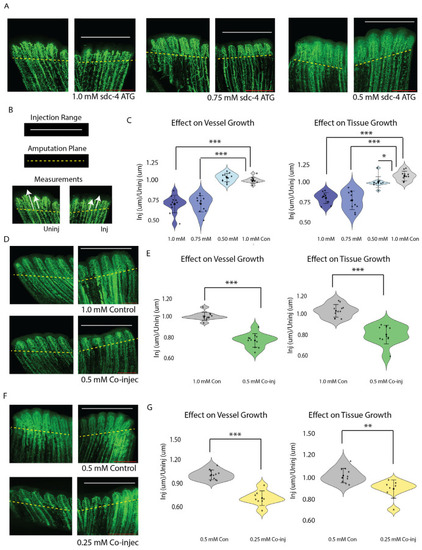
Combined subthreshold morpholinos |
|
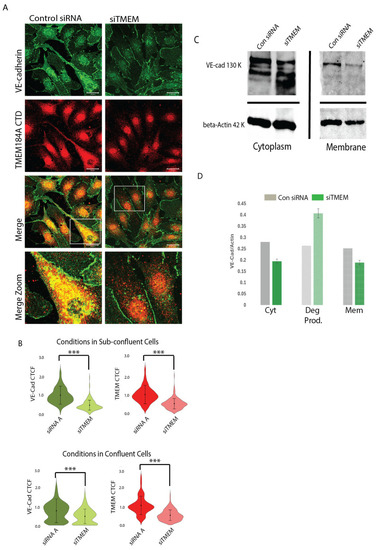
TMEM184A siRNA KD decreases post-translational levels of VE-cad in the membrane and in cytoplasmic vesicles. ( |
|
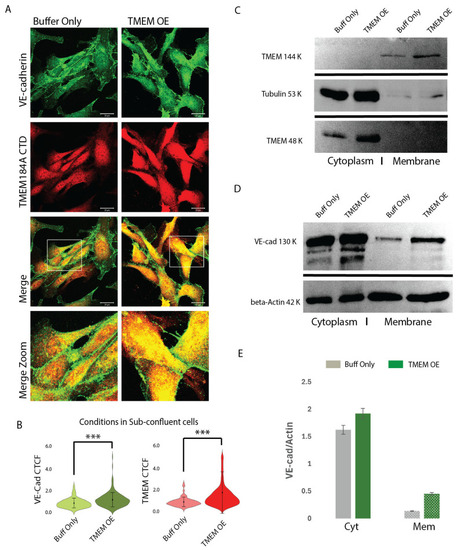
TMEM OE increases VE-cad rapid recycling and membrane levels. ( |
|
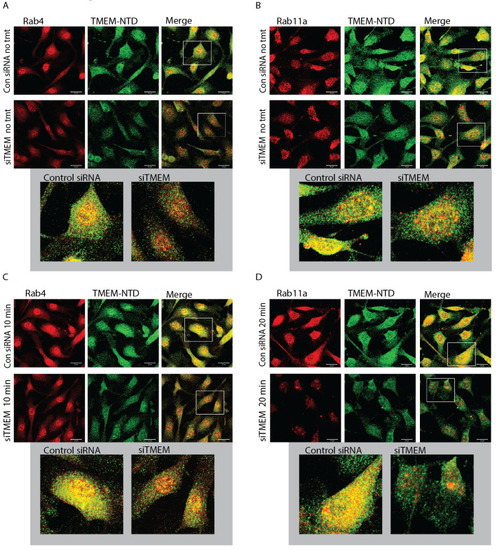
TMEM184A colocalizes with recycling Rab-GTPases in the presence of VEGF. BAOECs were treated with VEGF-165 or left untreated as noted, fixed, and permeabilized with 0.5% Triton X-100 to increase the visibility of vesicles. ( |
|
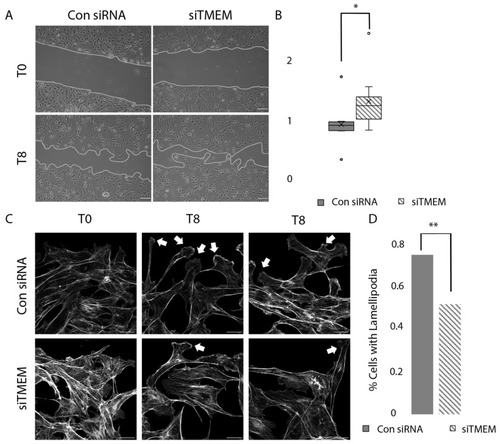
Rate of closure in siTMEM and siRNA cells. ( |